Silver Density Guide: Accurate G/Cm3 Values
Silver, known for its high electrical conductivity, malleability, and ductility, is a highly valued metal in various industries, including electronics, jewelry, and coins. One of the key physical properties of silver that is crucial for its applications is its density. Density, measured in grams per cubic centimeter (g/cm^3), is a fundamental characteristic that defines how much mass is contained in a given volume of a substance. Understanding the density of silver is essential for calculating volumes, weights, and for ensuring the quality and authenticity of silver products. This guide provides an overview of the density of silver, its variations, and the importance of accurate density measurements in industrial and commercial applications.
Introduction to Silver Density

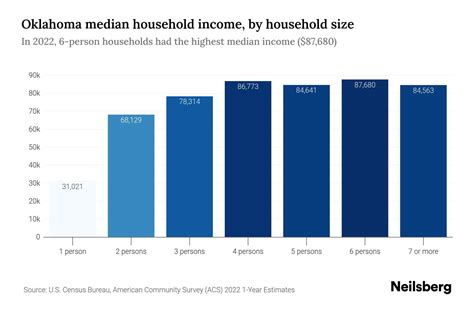
The density of silver is a well-defined physical constant. At standard temperature and pressure (STP) conditions, which are defined as 0 degrees Celsius (273.15 Kelvin) and 1 atmosphere (101.325 kPa), the density of pure silver is approximately 10.49 g/cm^3. This value is critical for various applications, including the production of silver alloys, where the addition of other metals can alter the density. For instance, sterling silver, an alloy containing 92.5% silver and 7.5% copper, has a slightly lower density due to the presence of copper, which has a density of about 8.96 g/cm^3.
Variations in Silver Density
While the density of pure silver is a fixed value under standard conditions, variations can occur due to several factors, including temperature changes and the presence of impurities or alloying elements. Temperature affects the density of silver because metals expand when heated and contract when cooled. The coefficient of thermal expansion for silver is approximately 18.9 x 10^-6 K^-1, meaning that for every degree Kelvin increase in temperature, silver expands by about 18.9 parts per million. This thermal expansion can slightly decrease the density of silver at higher temperatures.
| Material | Density (g/cm^3) |
|---|---|
| Pure Silver | 10.49 |
| Sterling Silver (92.5% Ag, 7.5% Cu) | 10.36 |
| Silver Alloy (with other metals) | Varies |
Measurement of Silver Density
Measuring the density of silver involves determining the mass of a known volume of the material or vice versa. The most common method is the Archimedes principle, which states that the buoyancy force on an object submerged in a fluid is equal to the weight of the fluid that the object displaces. By measuring the weight of the silver sample in air and then in water (or another fluid of known density), one can calculate the volume of the sample and hence its density. Other methods, such as using a densitometer or calculating from the dimensions and mass of a regularly shaped sample, can also be employed.
Importance of Accurate Density Measurements
Accurate density measurements are vital for several reasons. In the field of electronics, where silver is used for contacts and conductors, knowing the exact density can help in calculating the required volume of silver for a specific application, ensuring optimal performance and minimizing material waste. In the jewelry industry, verifying the density of silver alloys is a common method for detecting counterfeit or adulterated products, as the density can indicate the presence of less dense metals. Furthermore, in scientific research, precise density values are essential for characterizing the properties of new silver-based materials and understanding their potential applications.
How does the density of silver compare to other metals?
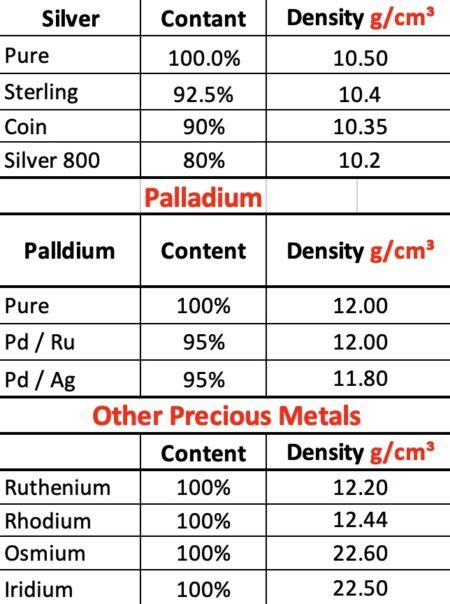
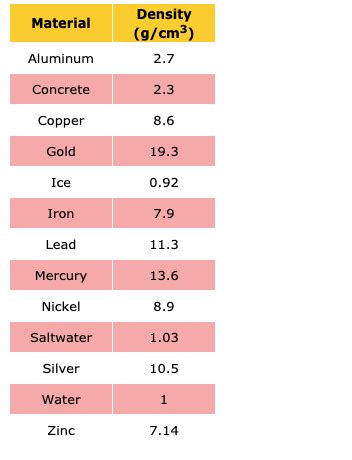
+Silver has a density that is higher than many other metals, such as copper and aluminum, but lower than metals like lead and tungsten. Its density is comparable to that of other precious metals like gold, which has a density of about 19.3 g/cm^3.
What are the implications of incorrect density measurements in silver applications?
+Incorrect density measurements can lead to miscalculations in material quantities, affecting the performance, durability, and authenticity of silver products. This can result in financial losses, damage to reputation, and in some cases, safety hazards.
In conclusion, understanding and accurately measuring the density of silver is fundamental for its various applications. The density of silver, approximately 10.49 g/cm^3, is a critical property that influences its use in electronics, jewelry, and other industries. Variations in density due to temperature, alloying, and impurities must be considered to ensure the quality and authenticity of silver products. As research and development continue to explore new applications and properties of silver, the importance of precise density measurements will only continue to grow.