Hydrogen Bonding Distance Explained
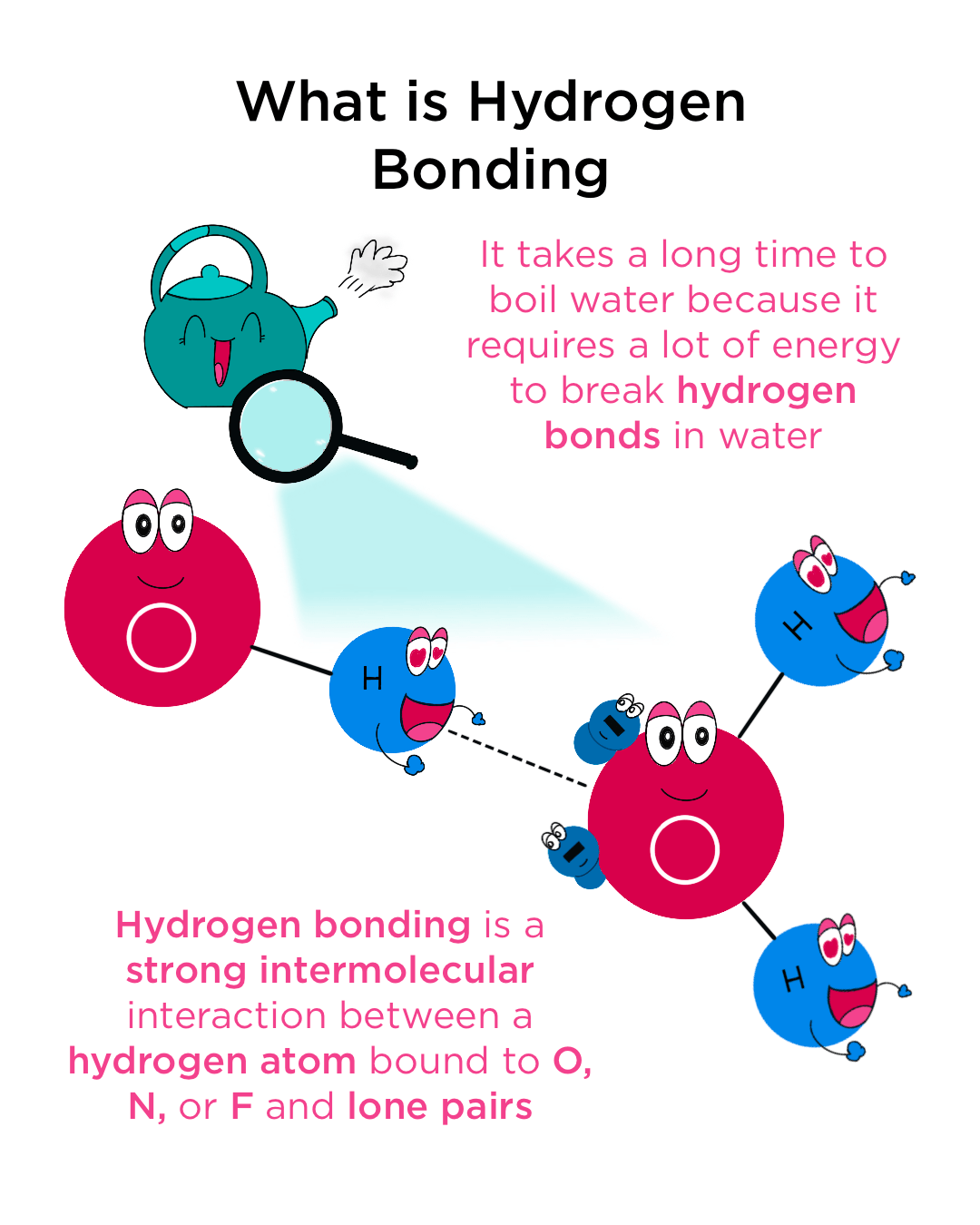
Hydrogen bonding is a fundamental concept in chemistry, playing a crucial role in the structure and properties of molecules. It is a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. The distance between the hydrogen atom and the electronegative atom in another molecule is known as the hydrogen bonding distance. This distance is critical in determining the strength and stability of the hydrogen bond.
The hydrogen bonding distance is typically measured in angstroms (Å) or picometers (pm). It is defined as the distance between the nucleus of the hydrogen atom and the nucleus of the electronegative atom in another molecule. The average hydrogen bonding distance varies depending on the type of molecules involved and the environment in which they exist. For example, in water molecules, the average hydrogen bonding distance is around 1.85 Å, while in ammonia molecules, it is approximately 2.0 Å.
Factors Influencing Hydrogen Bonding Distance

The hydrogen bonding distance is influenced by several factors, including the electronegativity of the atoms involved, the polarity of the molecules, and the temperature and pressure of the environment. Electronegativity plays a significant role in determining the hydrogen bonding distance, as it affects the distribution of electrons within the molecule. Molecules with highly electronegative atoms, such as oxygen or fluorine, tend to form stronger hydrogen bonds with shorter distances.
Hydrogen bond strength is another critical factor that influences the hydrogen bonding distance. Stronger hydrogen bonds result in shorter distances, while weaker bonds lead to longer distances. The strength of a hydrogen bond is determined by the polarity of the molecules involved and the orientation of the molecules with respect to each other. In general, molecules with higher polarity and optimal orientation tend to form stronger hydrogen bonds with shorter distances.
Hydrogen Bonding Distance in Different Molecules
The hydrogen bonding distance varies significantly in different molecules. For example, in water molecules, the average hydrogen bonding distance is around 1.85 Å, while in ammonia molecules, it is approximately 2.0 Å. In hydrogen fluoride molecules, the hydrogen bonding distance is even shorter, around 1.5 Å, due to the high electronegativity of the fluorine atom.
| Molecule | Hydrogen Bonding Distance (Å) |
|---|---|
| Water (H2O) | 1.85 |
| Ammonia (NH3) | 2.0 |
| Hydrogen Fluoride (HF) | 1.5 |
| Methanol (CH3OH) | 1.9 |
| Formic Acid (HCOOH) | 1.7 |
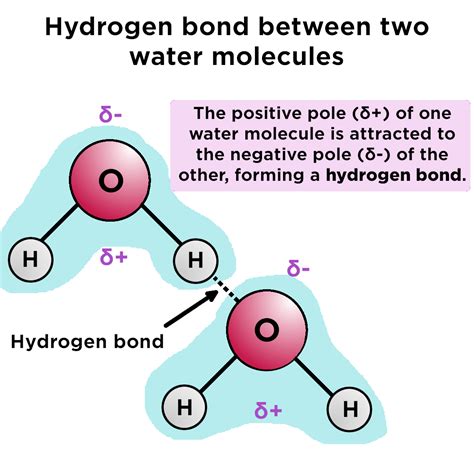
Importance of Hydrogen Bonding Distance in Biological Systems

In biological systems, hydrogen bonding distance plays a vital role in determining the structure and function of biomolecules, such as proteins, DNA, and RNA. The double helix structure of DNA, for example, is stabilized by hydrogen bonds between the nucleotide bases, with an average distance of around 2.9 Å. Similarly, the secondary structure of proteins is influenced by hydrogen bonds between the amino acids, with distances ranging from 1.5 to 2.5 Å.
The strength and stability of these hydrogen bonds are critical in maintaining the native conformation of biomolecules and facilitating their biological functions. Changes in the hydrogen bonding distance can lead to significant alterations in the structure and function of biomolecules, which can have profound effects on the overall health and well-being of an organism.
Hydrogen Bonding Distance in Protein-Ligand Interactions
In protein-ligand interactions, hydrogen bonding distance plays a crucial role in determining the binding affinity and specificity. The distance between the hydrogen bond donor and acceptor atoms influences the strength of the interaction, with shorter distances resulting in stronger bonds. The orientation of the hydrogen bond is also critical, with optimal orientation leading to more stable interactions.
Understanding the hydrogen bonding distance in protein-ligand interactions is essential in the design of new drugs and therapies, where the control of hydrogen bonding interactions can lead to improved efficacy and selectivity.
What is the average hydrogen bonding distance in water molecules?
+The average hydrogen bonding distance in water molecules is around 1.85 Å.
How does the electronegativity of atoms influence the hydrogen bonding distance?
+The electronegativity of atoms plays a significant role in determining the hydrogen bonding distance. Molecules with highly electronegative atoms, such as oxygen or fluorine, tend to form stronger hydrogen bonds with shorter distances.
What is the importance of hydrogen bonding distance in biological systems?
+Hydrogen bonding distance plays a vital role in determining the structure and function of biomolecules, such as proteins, DNA, and RNA. It influences the strength and stability of hydrogen bonds, which is critical in maintaining the native conformation of biomolecules and facilitating their biological functions.



